Study of hydrogen transfer reactions in biological enzymes
Hydrogen transfer reactions play a significant role in many organic and biological reactions. Due to the de Broglie wavelength of the transferring hydrogen atom, the role of quantum nuclear effects in such reactions have been one focus area of study. Experimentally, an important indication of quantum nuclear effects including tunneling is the appearance of an unexpectedly large primary kinetic isotope effect (KIE), which has been noted in many lipoxygenases. For example, the room temperature rate constant for hydrogen nuclear transfer ($k_H$) catalyzed by the enzyme soybean lipoxygenase-1 (SLO-1) is a factor of 81 larger than that for deuterium nuclear transfer (k_D). Human lipoxygenase was noted to have a similar behavior. Quantum mechanical tunneling has been proposed to have a central role in this phenomenon, since this observation cannot be explained using classical rate theories. Temperature dependence of primary and secondary isotope effects are another set of experimentally measurable parameters that directly probe the extent of quantum nuclear effects. The proper description of nuclear quantum effects for hydrogen-transfer reactions, including the role of tunneling, is a challenging and an often actively debated area of study.
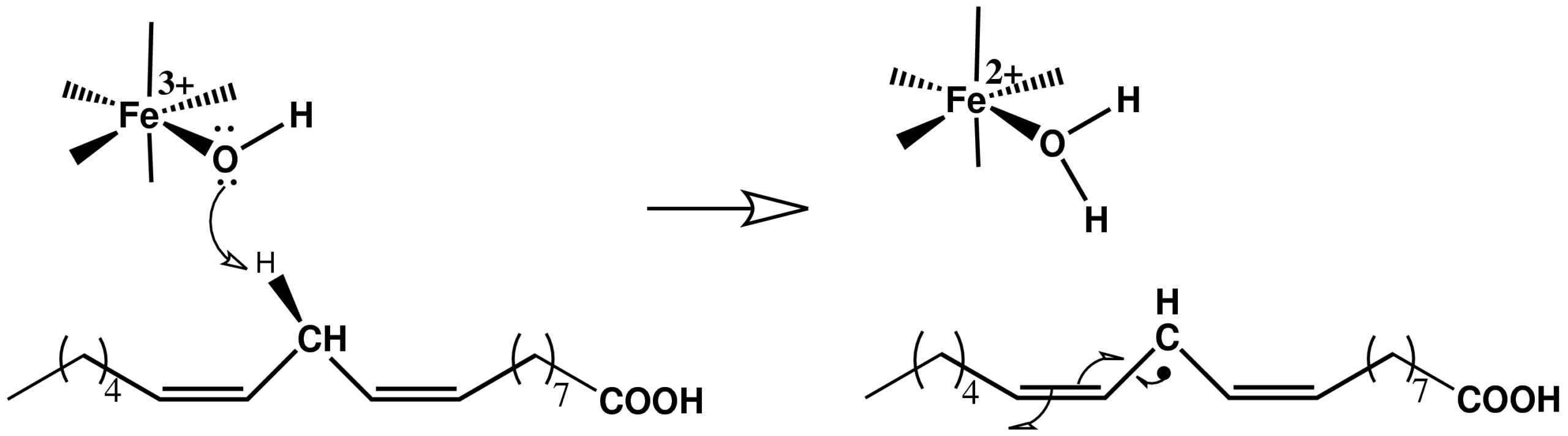
In recent publications we explored the hydrogen and deuterium nuclear tunneling process involved in the rate-determining step in the catalytic cycle of the enzyme SLO-1. This step [see figure] involves the abstraction of a hydrogen atom from the substrate [linoleic acid] by the octahedral Fe$^{3+}$-OH complex present deep in the active site The reaction displays a large KIE [$k_H/k_D$] of 81 at room temperature under certain mutations. We computed the hydrogen tunneling probabilities for a model system constructed from the active site atoms in close proximity to the iron cofactor in SLO-1. This simplification of the active site is based on the assumption that only the immediate environment exerts an electronic influence on the hydrogen nuclear transfer. We described the tunneling hydrogen nucleus [proton or deuteron] as a three-dimensional quantum wavepacket coupled to the change in electronic structure which was computed using hybrid density functional theory, benchmarked with MP2 post-Hartree-Fock theory. At each step of the quantum dynamics, the potential surface was computed by including all electrons in our model system. As a result, our method is not restricted to a specific mode of transfer such as proton coupled electron transfer, proton transfer, hydrogen transfer or hydride transfer.


 N. DeGregorio and S. S. Iyengar, "Challenges in constructing accurate methods for hydrogen transfer reactions in large biological assemblies: rare events sampling for mechanistic discovery and tensor networks for quantum nuclear effects", Faraday Discussions 221, 379-405 (2019).
N. DeGregorio and S. S. Iyengar, "Challenges in constructing accurate methods for hydrogen transfer reactions in large biological assemblies: rare events sampling for mechanistic discovery and tensor networks for quantum nuclear effects", Faraday Discussions 221, 379-405 (2019).
 The College of Arts
The College of Arts